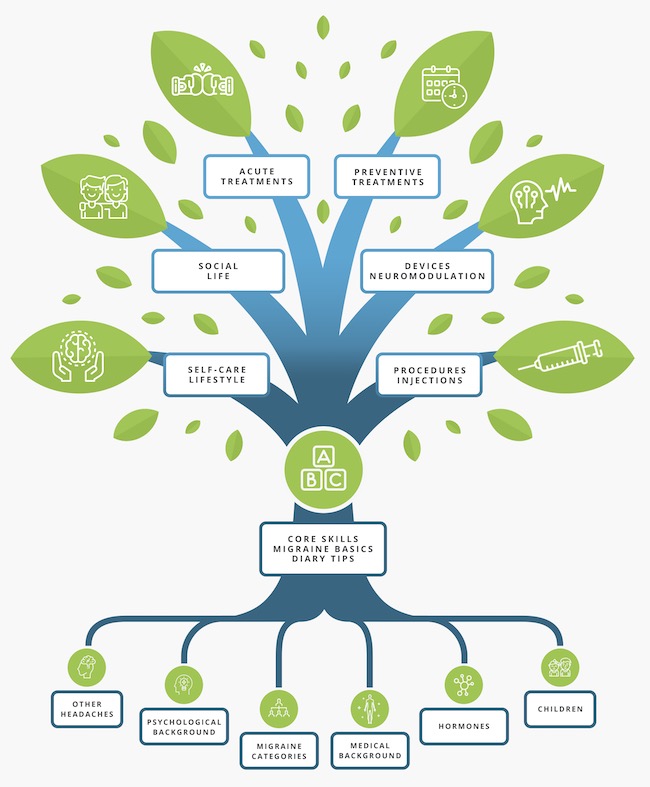
The Migraine Tree
All you need to know about migraine!
The Migraine Tree Post of the week:

List of migraine preventives: classes and mechanism of action
«What’s the best preventive for migraine»? Sadly, there is no answer to this question. Many different approaches exist, and it is not possible to predict which one will work for one person in particular. For each option, there are people who do not improve (non-responders),...
Our Latest Posts
CADTH supports the reimbursement of Botox for Chronic Migraine
Good News! CADTH supports the reimbursement of Botox for Chronic Migraine Botox was approved by Health Canada in 2011, but reimbursement by public payers was difficult to obtain. Following an updated recommendation from CADTH, access is likely to improve for Canadians with Chronic Migraine. What…
Another new drug for migraine is likely on its way to Canada.
A completely new type of migraine preventive medication has been developed over the last ten years, one which uses antibodies to stop migraine attacks. These antibodies are similar to the ones that help you fight viruses, like the common cold, but they are made to…
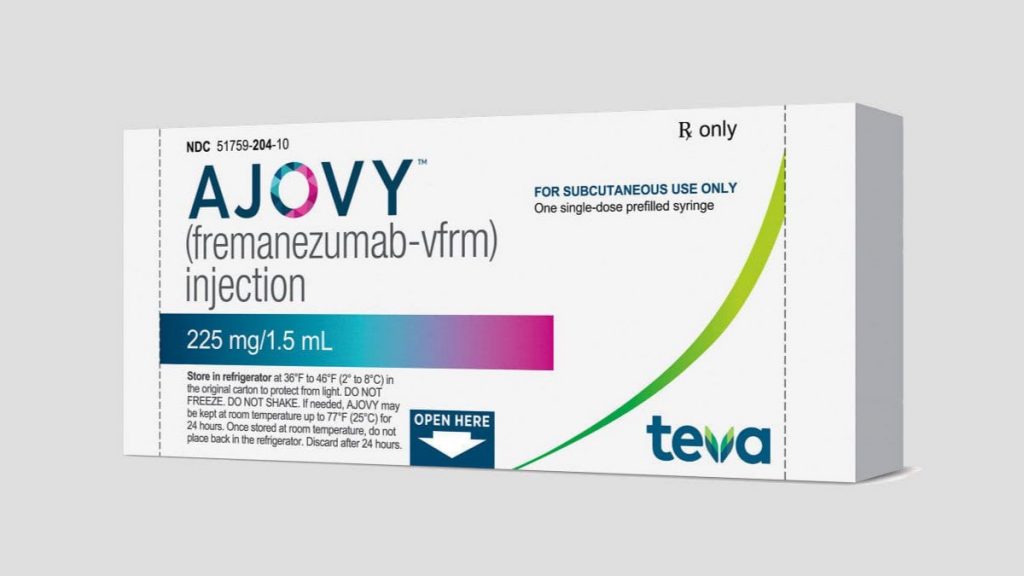
Ajovy (fremanezumab) approved by Health Canada
As of April 9th, Health Canada has approved Ajovy for use in Canada. This is great news for people living with migraine! ** ALWAYS discuss with your health care provider when making decisions about medications. What is Ajovy? Ajovy is a monoclonal antibody targeting CGRP….